Description
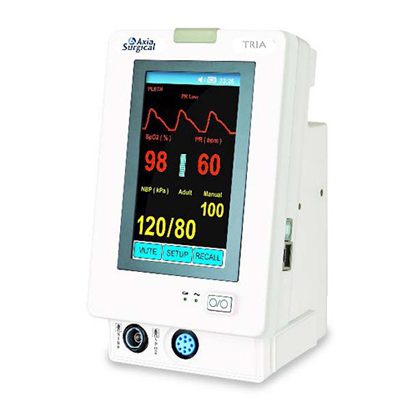
Axia TRIA Touch Screen Patient Monitor
The Axia TRIA is a new and intuitive approach to patient vital signs measurements. The TRIA can be configured to measure any combination of: non-invasive blood pressure, SpO2, rapid temperature and capnography (EtCO2).
The TRIA is well suited for any patient care area by offering a multitude of vital sign combinations. The TRIA can be used as a basic pulse oximeter or configured to a NIBP / SpO2 / Temp spot check monitor. The TRIA can also be configured to be a stand alone capnograph or a combination capnograph SpO2 / NIBP monitor. The TRIA is well suited for bed side and mobile spot check use.
- Touch screen
- Simple interface
- Intuitive
- Long life Lithium Battery
- Mobile (Backup battery powered)
- Portable (weight 1.71 lbs)
- Color waveforms
- Applications: Neonatal, pediatric and adult patients
Axia TRIA Touch Screen Specifications
Dimensions
- Size: 8 x 4.5 x 4 (HxWxD inches)
- Weight: 2.5 LBS
Performance Specifications
- Display: 5.0 inch (Diagonal) color TFT
- Resolution: 800 × 3(RGB) × 480
- Trace: 2 waveforms
- Waveforms: PLETH, ETCO2
- Indicator: Alarm Indicator, Power indicator, Pulse beep and alarm sound
- Trend time: From 1 to 72 hours
SPO2
- ASpO2: Anti-motion SpO2
- SpO2% Range: 0 ~ 100%
- SpO2 Accuracy: ±2% (70 ~ 100%,non-motion), ±3% (70 ~ 100%, motion)
- Pulse Rate Range: 30-250 bpm
- Pulse Rate Accuracy: ±2 bpm(non-motion), ±3 bpm (motion)
- Alarm Upper-lower Limit: Upper limit 70 ~ 100%, Lower limit 70 ~ 100% bpm (motion)
- SpO2 Probe:Red light LED wavelength: 660nm±5nm, Infrared light LED wavelength: 940nm±10nm
- Standards: Meets performance of EN ISO 9919:2015
NIBP
- Measuring Technology: Automatic oscillating measurement
- Cuff Inflating: <30s (0 ~ 300 mmH, standard adult cuff)
- Measuring Period: AVE<40s
- Mode: Manual, Auto, STAT
- in AUTO Mode: 2 min ~ 4 hrs
- Pulse Rate Range: 30 bpm ~ 250 bpm
- Measuring Range: Adult/Pediatric Mode, SYS: 40 ~ 250 (mmHg),DIA: 15 ~ 200 (mmHg), Neonatal Mode, SYS: 40 ~ 135 (mmHg), DIA: 15 ~ 100 (mmHg)
- Resolution:1mmHg
- Pressure Accuracy: Maximum Mean error: ±5mmHg
- Deviation: 8mmHg
- Overpressure Protection: Adult Mode: 280(mmHg), Neonatal Mode: 150 (mmHg)
- Alarm Limit: SYS: 50 ~ 240 mmHg, DIA: 15 ~ 180 mmHg
- Standards: Meets performance standards ANSI/AAMI SP10:2002
ETCO2(OPTION)
- Mode of Sampling: Sidestream or Mainstream
- Principle of Operation: Non-dispersive infrared (NDIR) single beam optics, dual wavelength, no moving parts.
- CO2 measurement Range: 0 to 150 mmHg (0 to 19.7%, 0 to 20 kPa)
- CO2 Calculation Method: BTPS (Body Temperature Pressure Saturated)
- CO2 Resolution: 0.1mmHg (0-69mmHg),0.25mmHg (70-150mmHg)
- CO2 Accuracy: 0 ~ 40 mmHg ± 2 mmHg, 41 ~ 70 mmHg ± 5% of reading, 71 ~ 100 mmHg ± 8% of reading, 101 ~ 150 mmHg ± 10% of reading, Above 80 breath per minute ± 12%of reading
- Sampling rate:100Hz
- Respiration Rate: 2 ~ 150 bpm
- Respiration Rate accuracy: ±1 breath
- Response Time: <3 seconds -includes transport time and rise time
- Measurement Range: 3 ~ 50 mmHg
- Standards: Meets performance standards of ISO/ FDIS 21647:2004 (E)
Networking
- Wired Networking:Industry standard: 802.11b/g wired network, Frequency Range: 2.412 ~ 2.484 GHz Connected bedside number: Up to 16 bedside monitors
- Wireless Networking: Up to 100m indoors, Industry standard 802.11b/g wireless Supports TCP/IP and UDP/IP Protocols
Power
- Source: External AC power or internal battery
- AC Power: 100 ~ 240VAC, 50/60Hz, 150VA
- Battery: Built-in and lithium Ion rechargeable, 12.6V/5Ah
- Charge Time: 8 hours
- Operating Time: 3 hours
Fuse
- 3.15A/250V
LCD Specifications
- Display Type: TFT color LCD
- Size (diagonal): 5.0 inch
- Active Area: 152.4 (W) × 91.44 (H) mm
- Color arrangement: RGB-stripe
- Dot pitch: 0.0635(W) × 0.1905(H) mm
- Display Mode: Normally white, Transmissive
- Interface: Digital (TTL)
- Surface: Treatment: Anti-Glare
Touchscreen Specifications
- Type: Four-Wire Analog Resistive Touch Panel
- Input Mode: Stylus Pen or Finger
- Connector:FPC
- Insulation resistance: 25MΩ
- : 7VDC
- Chattering: 10ms
- Transparency: 80%
- Surface hardness: 3H
- Durability-surface scratching: Write 100,000
- Active force: 80gf
- Knock Test: 1,000,000 times
Rapid Temperature (Option)
- Measurement Range: 30°C to 43°C (86°F to 109°F)
- Typical Measurement Times: Oral (Quick Mode): 3-5 seconds (non-fever temps)
- (after insertion into measurement site): 8-10 seconds (fever temps), Oral (Standard Mode): 6-10 seconds Axillary Mode: 8-12, Rectal Mode: 10-14 seconds, Direct Mode (All Sites): 60-120 seconds
- Pulse Timer: 60 Second count with a “beep” at 15 seconds, 2 “beeps” at 30 seconds, 1 “beep” at 45 seconds, and 2 “beeps”at 60 seconds
- Patient Accuracy: A Standard Prediction Mode reading and a Direct Mode reading will differ by less than ±0.2°C (±0.4°F) on 98% of tested patients
- Batteries: Four “AA” Required, Standard IEC package size. Alkaline –1.5 Volt, Approx. 6000 temperature readings
- Standards: Meets performance standards of EN 12470-3:2000 and ASTM E1112:2006
Safety
- Meet the requirement of EN60601 series, CE marking according to MDD93/42/EEC
- Type of Protection: Class I (on AC power) , internally powered equipment (on battery power):Per I.E.C. 60601-1, clause 2.2.4
- Degree of Protection: Type BF, defibrillator-proof CF – Applied part
- Sterilization or Disinfection methods: 70% isopropyl alcohol solution or a non staining disinfectant. Equipment not suitable for use in the Presence of a flammable anesthetic mixture with air or with oxygen or nitrous oxide
- Operation Mode: Continuous
- Protection Against Ingress of Liquids: IPXO